2013 Press Releases
Scroll down to see each press release:October 29 2013 Chronic Pelvic and Bladder Pain Patients Have New Non-Surgical, Opioid-Free Treatment Option with Wearable Ultrasound Device, Confirms Study Extending Earlier Findings
Significant reduction of persistent, difficult-to-treat pelvic and interstitial cystitis pain using a new therapeutic wearable ultrasound device was found at long-term follow-up, extending earlier findings in patients with chronic pelvic, bladder, genital, bowel and musculoskeletal pain. The study by the International Adhesions Society has been accepted for presentation at the prestigious AAGL Global Congress on Minimally Invasive Gynecology in Washington, DC Nov 13, 2013.
Elizabeth, 46 PainShield MD User
![]() I have reduced the amount and cost of my pain medications,
and my pain is reduced drastically so that I am able once again to sleep
through a full night.
I have reduced the amount and cost of my pain medications,
and my pain is reduced drastically so that I am able once again to sleep
through a full night.![]()
Dallas, TX (PRWEB) October 29, 2013
Extending the findings of an earlier clinical study, long-term data to be presented at the prestigious AAGL Global Congress on Minimally Invasive Gynecology in Washington, DC Nov 13, 2013 demonstrated significant reductions in pain in 16 women who were followed for up to 541 days after suffering from long-standing, difficult-to-treat chronic pelvic pain, interstitial cystitis, adhesions and endometriosis.
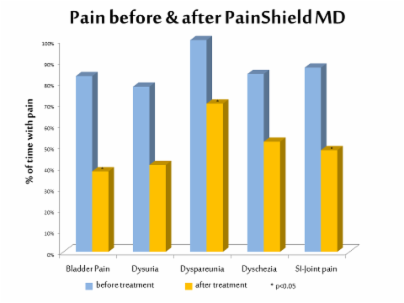
Patients used a wearable, patch-based therapeutic ultrasound device, PainShield® MD, for several hours each day, which produced clinically significant reductions in a variety of pain scores, including chronic pelvic and abdominal pain, bladder pain when urinating (dysuria), and muscle and joint pain. The device also significantly reduced the number of painful urination episodes, particularly at night-time, as well as intercourse pain (dyspareunia), sacroiliac joint pain, and painful bowel movements.
Many PainShield MD patients reported significant improvements in their quality of life such as better sleep, weight loss from improved activity, and lower consumption of pain and other medications including opioids. Not only were drug side-effects such as constipation reduced, but patients reported savings up to several hundred dollars a month in medication costs.
One patient, Elizabeth, 46, an oil field tools buyer from Texas, has been in pain for over 30 years with pelvic adhesive disease, endometriosis and eight major surgeries, including a hysterectomy, colectomy and an ileostomy. Elizabeth reports that PainShield MD “has reduced my bowel pain, my pelvic pain and my bladder pain… it has significantly reduced my sexual pain, and I can be a normal woman. I have reduced the amount and cost of my pain medications, and my pain is reduced drastically so that I am able once again to sleep through a full night.”
The PainShield MD clinical study resulted from a grassroots effort under the leadership of a patient advocacy organization, the International Adhesions Society (IAS), which provides information, support and advocacy to patients and their families suffering from adhesion related disorder (ARD). Authoring the study together with Dr. Wiseman was Ms. Teena Petree, PT of Summit Physical Therapy, Dallas, TX, whose specialties include pelvic pain relief. Although ultrasound has been used by physical therapists for many years, the wearable PainShield MD overcomes its limitations by extending the duration of therapy for patients when at home or on-the-go. Pelvic muscle spasm plays a major role in pelvic pain. Relieving these contractures for Interstitial Cystitis/Bladder Pain Syndrome is a fundamental clinical principle of the American Urological Association, directly addressed by PainShield MD which is believed to increase blood flow to muscles, reduce contractures and thereby decrease pain.
The International Adhesions Society was founded by David Wiseman, PhD who remarked, “After conducting research on adhesions and pelvic pain and working with patients suffering from these conditions for over 25 years, I feel that this treatment modality can revolutionize the management of pain in the chronic pelvic pain population without the risks of surgery or drugs." Wiseman added, “With FDA’s announcement last week on the reclassification of hydrocodone combination products (e.g. Vicodin) that will make it harder to obtain these sorts of painkillers, it is all the more important that chronic pain patients be given real alternatives. The results of our study confirm that PainShield MD is a ‘game changer’ in the treatment of chronic pelvic and abdominal pain and is a non-drug alternative for this patient population. We are now planning a larger study to obtain the necessary data to secure insurance reimbursement, a vital next step in making the technology available to millions of pain patients.”
About KevMed
KevMed LLC
is a medical company formed by Dr. Wiseman as a result of this work with
PainShield MD. KevMed is dedicated to the relief of chronic pelvic pain and
related disorders and markets PainShield® MD, a wearable therapeutic ultrasound
device as a non-invasive, low-risk treatment modality for chronic pelvic pain
and pain associated with related conditions.
PainShield MD is available by prescription to patients in the United States and was developed by NanoVibronix of Melville, New York, based on its proprietary technology which miniaturizes the ultrasound delivery system to a small disc mounted within an adhesive patch applied to the skin. NanoVibronix provided the PainShield MD units for the study free of charge.
For more information contact:
Dr. David Wiseman
International Adhesions Society
18208 Preston Road, Suite D9
PMB 405, Dallas TX 75248
http://www.adhesions.org
KevMed, LLC.
15305 Dallas Parkway, Suite 300
Addison, TX 75001
October 15 2013 New Ultrasound Device Significantly Reduces Pain in Chronic Pelvic and Interstitial Cystitis Pain Patients–Clinical Study Presented to IPPS Extends Earlier Findings
A study following patients for up to 17-months of treatment with a new therapeutic, wearable ultrasound device for long-standing, difficult-to-treat pelvic and interstitial cystitis pain extended earlier findings that the device dramatically reduces severe and persistent chronic pelvic, bladder, genital, bowel and musculoskeletal pain. More than 15 million patients in the United States suffer from chronic pelvic, bladder and genital pain, endometriosis, and adhesions with few effective non-drug treatment options available. New Ultrasound Device Significantly Reduces Pain in Chronic Pelvic and Interstitial Cystitis Pain Patients–Clinical Study Presented to IPPS Extends Earlier Findings
A study following patients for up to 17-months of treatment with a new therapeutic, wearable ultrasound device for long-standing, difficult-to-treat pelvic and interstitial cystitis pain extended earlier findings that the device dramatically reduces severe and persistent chronic pelvic, bladder, genital, bowel and musculoskeletal pain. More than 15 million patients in the United States suffer from chronic pelvic, bladder and genital pain, endometriosis, and adhesions with few effective non-drug treatment options available.
A clinical follow-up study to be presented at the International Pelvic Pain Society Annual Meeting in Orlando (Oct. 17-19, 2013) found significant reduction in pain and related symptoms in women who had previously suffered from long-standing, difficult-to-treat chronic pelvic pain, interstitial cystitis, adhesions and endometriosis. This study followed 16 women who were treated with the PainShield® MD Therapeutic Ultrasound device for up to 541 days, confirming and extending the findings of a previous report which followed these women, as well as three men for 207 days.
This latest study found that the PainShield® MD Therapeutic Ultrasound device produced clinically significant reductions in a variety of pain scores, including chronic pelvic and abdominal pain, bladder pain when urinating (dysuria), and muscle and joint pain. The device also significantly reduced the number of painful urination episodes, particularly at night-time, pain related to intercourse (dyspareunia), sacroiliac joint pain, and painful bowel movements. The study findings also include anecdotal reports of clinically significant reductions in pain medication usage, including opioids, and the resulting cost savings, as well as improvements in sleep due to less pain.
Fifteen out of sixteen patients rated their response to the treatment with PainShield MD as “Good” (9/16), “Moderate” (2/16) or “Mild” (4/16), with only one patient reporting a negative response with rapid onset of pain (within one day), which subsided rapidly with discontinuation of therapy. This patient reported similar reaction to conventional, office-based ultrasound previously.
Since the introduction of the PainShield MD, many users have reported significant improvements in their quality of life due to reduced pain, better sleep, weight loss from improved activity levels, reduced medication expenses, and fewer detrimental drug side-effects, such as constipation, due to lower medication consumption. One such patient, Dawn, 49, from Aurora, CO, a mother and grandmother of four, reports: “I LOVE my PainShield. Before I received my miracle machine, I spent the majority of my day lying down. I have had my PainShield MD since December 2012 and usually place it on my lower abdomen. I move it around based upon where the pain is located. I really like the flexibility of being able to place it where I need it most.” Dawn has resumed activities like walks with her husband, cooking and spending time outdoors with her grandchildren – all previously impossible due to the debilitating pain she has suffered for over ten years from adhesions that followed a burst appendix and multiple GYN surgeries.
The PainShield MD clinical study resulted from a grassroots effort under the leadership of a patient advocacy organization, the International Adhesions Society (IAS), which provides information, support and advocacy to patients and their families suffering from adhesion related disorder (ARD). Authoring the study together with Dr. Wiseman was Ms. Teena Petree, PT of Summit Physical Therapy, Dallas, TX, whose specialties include pelvic floor dysfunction and pelvic pain relief.
The International Adhesions Society was founded by David Wiseman, PhD who remarked, “after conducting research on adhesions and pelvic pain and working with patients suffering from these conditions for over 25 years, I feel that this treatment modality can revolutionize the management of pain in the chronic pelvic pain population without the risks of surgery or drugs." Added Wiseman, “The results in the original PainShield MD study were so impressive that I felt compelled to start a company, KevMed, to make this technology widely available to patients in the US. Together with nearly a year of impressive results from actual market experience, these latest study results confirm our earlier conclusion that PainShield MD is a ‘game changer’ in the treatment of chronic pelvic and abdominal pain. We are now planning a larger study to obtain the necessary data to secure insurance reimbursement, a vital next step in making the technology available to millions of pain patients.”
About KevMed
KevMed LLC is a medical company dedicated to the relief of chronic pelvic pain and related disorders. KevMed markets PainShield® MD, a wearable therapeutic ultrasound device as a non-invasive, low-risk treatment modality for chronic pelvic pain and pain associated with related conditions. PainShield MD is available by prescription to patients in the United States and was developed by NanoVibronix of Melville, New York, based on its proprietary technology which miniaturizes the ultrasound delivery system to a small disc mounted within an adhesive patch applied to the skin. NanoVibronix provided the PainShield MD units for the study free of charge.
For more information, contact:
David Wiseman, PhD
International Adhesions Society
18208 Preston Road, Suite D9
PMB 405, Dallas TX 75248
http://www.adhesions.org
KevMed, LLC.
15305 Dallas Parkway, Suite 300, Addison, TX 75001
http://www.kevmed.com
________________________________________________________
April 23 2013 KevMed Co-Sponsors Unique Educational and Therapeutic Retreat for Pelvic Pain Sufferers hosted by the Alliance for Pelvic Pain this Weekend
Women with chronic pelvic, bladder, sexual, and genital pain will be empowered, enlightened and enlivened this coming weekend of April 27-28 at a unique educational retreat offering seminars, interactive workshops, one-on-one attention and treatment by leading health care specialists in chronic pelvic pain and related complex conditions such as Interstitial Cystitis (IC), Irritable Bowel Syndrome (IBS), pelvic floor dysfunction, vulvodynia, vestibulitis, lichen sclerosis, pudendal neuralgia, endometriosis, and other pelvic, genital, and sexual pain disorders. This event is hosted by the Alliance for Pelvic Pain, a multi-disciplinary group of leading healthcare practitioners, and KevMed will be among the co-sponsors of this event, exhibiting the “game-changing” PainShield® MD Therapeutic Ultrasound while sharing recent clinical data and patient successes on using this wearable, non-invasive nano-technology device to alleviate pelvic pain.
Dallas, TX (PRWEB) April 23, 2013
KevMed, provider of the PainShield® MD Therapeutic Ultrasound device, will be among the co-sponsors at this weekend’s first-of-its-kind educational retreat for pelvic pain sufferers, hosted at the historic Bethlehem Hotel in Bethlehem, PA by the Alliance for Pelvic Pain, April 27-28, 2013. Founded by leading specialists in the field of pelvic pain, including Robert J. Echenberg, MD, Alexandra T. Milspaw, MEd, LPC, Deborah Coady, MD, Amy Stein, MPT, and Nancy Fish, MSW, the Alliance for Pelvic Pain was created to foster a multi-disciplinary approach to the clinical care, education, and research associated with pelvic pain. The retreat, aptly named “Connecting the Dots of Your Experience,” will give patients a solid foundation of knowledge, skills, and comprehensive treatment modalities to address their chronic pelvic pain. While registration for the retreat is officially closed, a very limited number of spaces remain for last-minute attendees.
Dr. Robert Echenberg, co-founder of the Alliance for Pelvic Pain and one of the speakers at this weekend’s retreat, is also the founder of the Institute for Women in Pain. While not originating the somewhat recent idea of a multi-disciplinary approach to pain, Dr. Echenberg has taken the concept to a new level by passionately offering insurance-based and compassionate care of a wide set of overlapping, coalescing pelvic, bladder, bowel and genital conditions via his team of multi-disciplinary, integrated and holistic professionals, such as the ones on hand at the upcoming seminar.
At the heart of this approach is an understanding that for patients suffering with seemingly unrelated pelvic, bladder, bowel and genital symptoms to be properly treated, they must be liberated from the constraints of compartmentalized medical practice. Indeed, by using in the title of his recent webinar “IC’s Role in CAPPS” the term CAPPS (Complex Abdominal Pelvic Pain Syndrome), which was coined by the International Adhesions Society, to do just that, Dr. Echenberg and his team may well be among the founders of a revolution in the treatment of a set of conditions affecting over 25 million Americans.
Says Dr. David Wiseman, founder of the International Adhesions Society (IAS) and president of the recently launched company KevMed, whose PainShield® MD will be exhibited at the upcoming retreat, “We are delighted to co-sponsor this important multi-disciplinary meeting of some of the best practitioners in the field of pelvic pain. This retreat will expand the knowledge of all patients attending and offer advances in medical technology, new therapies, understanding, compassion, and above all, hope for pelvic pain sufferers.” Adds Dr. Wiseman, “this retreat represents a further step in the national expansion of the inter-disciplinary concept we have for some time been advocating and working towards with our efforts that include establishment of a CAPPS clinic and the development of PainShield MD. We wish the Alliance for Pelvic Pain much success and have every confidence that the event will help liberate CAPPS patients from the confusing bonds of compartmentalized medicine that have until now hindered their diagnosis and treatment.”
About KevMed
KevMed LLC is a medical company dedicated to the relief of chronic pelvic pain and related disorders. KevMed markets PainShield® MD,
a wearable therapeutic ultrasound device as a non-invasive, low-risk
treatment modality for chronic pelvic pain and pain associated with
related conditions. KevMed was born from the work of the International
Adhesions Society (IAS), which provides information, support, and
advocacy to patients and their families suffering from Adhesion Related
Disorder (ARD) and Complex Abdominal and Pelvic Pain Syndrome (CAPPS).
Research by the IAS has revealed new understandings of these and related
conditions from the perspective of the patient, resulting in the
identification of PainShield® MD as an effective treatment for pelvic
pain. KevMed, the IAS and the International Society for CAPPS
were founded by David Wiseman PhD, MRPharmS, an internationally
recognized expert in the science and business of adhesions, who has been
making important product and scientific contributions to the field
since 1987, most recently with Synechion, a consulting company he
founded in 1996.
Contact:
Dr. David Wiseman, President
KevMed™, LLC
15305 Dallas Parkway, Suite 300, Addison, TX 75001
972 931 5596
david.wiseman@kevmed.com
________________________________________________________
March 31 2013 KevMed supports study of opioid use in chronic pain patients by International Adhesions Society submitted to FDA challenging proposed opioid policy
KevMed is proud to have supported the study described in the following press release from the International Adhesions Society:
Chronic Pain Patients, Concerned About National Cold Turkey, Submit Own Data Challenging FDA’s Proposed Opioid Policy
Supported by its own specifically conducted research with 2909 patients from 10 patient groups, the International Adhesions Society (IAS) today submitted recommendations in response to FDA proposals to limit the the way opioids are approved. The data, representing over 25 million Americans with chronic pelvic, abdominal or spinal pain due in part to adhesions, endometriosis, interstitial cystitis, irritable bowel syndrome and hysterectomy complications, demonstrate the significant number of patients (92.4%) whose opioid requirements would fall outside of the proposed limits and whose access to opioids would be compromised. Chronic Pain Patients, Concerned About National Cold Turkey, Submit Own Data Challenging FDA’s Proposed Opioid PolicySupported by its own specifically conducted research with 2909 patients from 10 patient groups, the International Adhesions Society (IAS) today submitted recommendations in response to FDA proposals to limit the the way opioids are approved. The data, representing over 25 million Americans with chronic pelvic, abdominal or spinal pain due in part to adhesions, endometriosis, interstitial cystitis, irritable bowel syndrome and hysterectomy complications, demonstrate the significant number of patients (92.4%) whose opioid requirements would fall outside of the proposed limits and whose access to opioids would be compromised.
Dallas, TX (PRWEB) March 31, 2013
The International Adhesions Society (IAS), focusing on chronic pain, today submitted recommendations, in response to FDA solicitations regarding proposals intended to curb the epidemic of opioid abuse and misuse by limiting how these drugs are approved.
“Most comments to FDA indirectly answered their questions,” notes Dr. David Wiseman, Founder of the IAS. “We've answered FDA’s questions directly with this study.”
The data, summarized by video, and representing over 25 million Americans with chronic pelvic, abdominal or spinal pain due partly to adhesions, endometriosis, interstitial cystitis, IBS and hysterectomies, demonstrate the significant proportion of patients with non-cancer pain (92.4%) whose opioid requirements would fall outside the proposed limits, compromising their access to opioids.
85% of patients have been taking opioids for much longer than the proposed 90 day limit, 55% for more than 2 years. Limiting the approval of opioids to severe pain would exclude 46% of subjects. 24% of patients taking higher doses of opioids would fall outside the proposed limits.
88% of patients were concerned that, even with "off-label" prescribing, the proposals would limit access to pain medication, because doctors would be less willing to prescribe, and/or payors would be less willing to reimburse.
Chronic pain affects over 100 million Americans, costing over $560 billion annually in direct medical expenses and productivity, in addition to an inestimable human cost. Relief by opioids comes at the medical, economic and tragic human cost of addiction and overdose.
The IAS statement echoes others that the proposals lack scientific basis and are unlikely to succeed. Further, certain aspects of FDA’s efforts to combat abuse and misuse fall beyond its mission, and the problem should be tackled by other measures and by a coordinated strategy, apparently lacking, involving FDA and other national, state and local agencies. This effort must be part of a wider strategy on pain under draft by the IPRCC.
The IAS is most concerned about the statement by FDA that it and other policymakers are “striving to find a balance between minimizing opioid drug abuse and misuse, while simultaneously enabling appropriate access to pain-relieving drugs.” Notes Dr. Wiseman, “this statement demonstrates our societal addiction to drugs by assuming that: a) opioids are the analgesics of choice and b) drugs are the treatment of choice for pain.” He adds, “alternatives, such as non-opioid analgesics, medical devices, physical therapy and psychotherapy need to be more widely available and reimbursed and be given fast-track approval. For many reasons we need to relegate opioids to a second line therapy. But until an integrated policy facilitates the use of alternatives to wean the nation carefully from opioids, we are concerned that the proposals may precipitate a national 'cold turkey' for millions of chronic pain patients and the war on opioid abuse and misuse is doomed."
Until alternatives to opioids like PainShield MD are more widely available, we need to ensure that chronic pain patients have access to opioid medication.”
Indeed, in recent research, the IAS identified and tested in a clinical study a novel wearable, non-invasive device, PainShield® MD Therapeutic Ultrasound, which reduced chronic bowel, bladder, intercourse, sacroiliac joint and other pain in patients with pelvic and abdominal pain, interstitial cystitis, IBS and pudendal neuralgia. Some patients were able to reduce or eliminate their use of opioids using this device. Impressed by these data, a company, KevMed, which sponsored the IAS study on opioids, was established to market PainShield MD for these conditions.
“Until alternatives to opioids like PainShield MD are more widely available, we need to ensure that chronic pain patients have access to opioid medication” notes Dr. Wiseman.
The IAS thanks the following for encouraging participation in this study: Arachnoiditis Society for Awareness and Prevention, drugwatch.com, EaseNervePain.com, Endometriosis Association, Endometriosis Research Center, HysterSisters, Interstitial Cystitis Association, Interstitial Cystitis Network, livinginpain.org. This assistance does not imply agreement with the IAS statement.
About the International Adhesions Society
The International Adhesions Society (IAS),
provides awareness, information, advocacy, support and research for
patients and their families suffering from adhesions - internal scars
that connect organs or tissues that are not normally connected, as well
as Adhesion Related Disorder (ARD) and Complex Abdomino-Pelvic Pain
Syndrome (CAPPS).
The IAS was founded and is funded by Synechion, Inc., a company
providing R&D consulting services focusing on adhesions. It is
expected that proceeds from KevMed will help to fund the efforts of the
IAS.
________________________________________________________
Jan 20 2013 Real-Life Success Story of Chronic Pelvic Pain Patient Portrays Journey of Recovery and Healing
A new 10-minute documentary video has
been released online, depicting the journey of a chronic pelvic pain
patient, Michelle Brown, 45, who has overcome 15 years of unbearable and
debilitating painful bladder and bowel symptoms related to her many
adhesions surgeries and her hysterectomy, with the use of a new wearable
therapeutic ultrasound device, PainShield® MD. The video, available at http://www.kevmed.com/Testimonials.html,
illustrates the life-changing effect of this novel device, which is a
non-invasive, low-risk treatment modality for chronic pelvic pain.
Dallas, TX (PRWEB) January 29, 2013
An inspiring 10-minute video has just been released by KevMed LLC, documenting a patient’s successful battle with debilitating chronic pelvic pain. Michelle Brown’s journey began in her late teens when her colon tore during surgery for a ruptured appendix, resulting in infection and adhesions (internal scar tissue), causing pain and bowel obstruction. With her condition worsening to include diagnoses of irritable bowel syndrome and fibromyalgia, Michelle, now 45, endured thirteen surgeries to remove adhesions and eventually a hysterectomy, all leaving her in ever increasing pain.
Trying all kinds of therapies, Michelle trekked across the USA and beyond to Germany, England and Mexico, to find specialists who could cure her chronic pelvic pain. Bladder pain and dysfunction required painful trips to the bathroom to urinate as often as 70 times per day, and at least hourly during the night, robbing her of much needed sleep. Bowel dysfunction from adhesions caused infrequent, extremely painful bowel movements, exacerbated by the side-effects of her many pain medications. Already limited in her ability to move without excruciating pain, the pain medications - costing between $300 and $1000 in out-of-pocket expenses monthly - also left Michelle lethargic and gaining 45 pounds. Michelle’s marriage suffered, as did her ability to drive, work, care for her two children, and lead an active, fulfilled life.
Michelle’s life changed in late 2010 shortly after enrolling in a clinical study being conducted by the International Adhesions Society to evaluate PainShield® MD, a novel wearable, non-invasive device delivering therapeutic ultrasound. Within just a few days of using the new device, Michelle noticed improvements in her bladder pain, her painful bowel movements started normalizing and she needed less pain medication. Six months of treatment, and Michelle had discontinued most of her pain medications, she could sleep through the night, bathroom visits no longer dominated her schedule, and she lost 36 pounds. “I’ve got so much more energy! I feel so much better!” beams Ms. Brown. “I can walk around more. I can do the games with the kids where you’re moving around,” adds Michelle.
Remarks Dr. David Wiseman, founder of the International Adhesions Society, “the results from the participation of Michelle and others in the PainShield® MD clinical study were so compelling and game-changing that we set up KevMed, LLC to market the device.” Results of the PainShield MD clinical study can be found on the KevMed website. PainShield® MD is believed to act by delivering therapeutic ultrasound energy to the pelvic muscles and other tissues, increasing blood flow, reducing spasms and reducing pain. Unlike conventional ultrasound, its miniature format means that its use is not limited to a therapist’s office or only 30 minutes of therapy at a time. The fact that PainShield MD can be worn for several hours at a time, almost anywhere, means that therapy can be provided over an extended period, maximizing its benefit.
Over 15 million patients in the US suffer from various overlapping disorders that include pelvic pain, abdominal pain, adhesions, bowel obstruction, endometriosis, interstitial cystitis (IC), painful bladder syndrome, irritable bowel syndrome (IBS), vulvodynia, dyspareunia (painful intercourse), dyschezia (painful defecation), sacroiliac joint pain, pudendal neuralgia and pelvic floor dysfunction. It is believed that PainShield MD will provide an important treatment for pain associated with these conditions.
Says David Wiseman, PhD, President and Founder of KevMed, “we are pleased to publish this inspiring video. Pelvic pain patients now have another option: non-invasive therapy they can administer at home, at work, even during sleep. “Adds Wiseman, “regaining control over one’s life is a key step toward recovery. Along with the other success stories of PainShield MD users we are posting on the KevMed web site, we hope this video will encourage and inspire pelvic pain sufferers.”
About KevMed
KevMed LLC is a medical company dedicated to the relief of chronic
pelvic pain and related disorders. KevMed was born from the work of the
International Adhesions Society (IAS), which provides information,
support, and advocacy to patients and their families suffering from
Adhesion Related Disorder (ARD) and Complex Abdominal and Pelvic Pain
Syndrome (CAPPS). Research by the IAS has revealed new understandings of
these and related conditions from the perspective of the patient,
resulting in the identification of PainShield® MD as an effective
treatment for pelvic pain. Both KevMed and the IAS were founded by David
Wiseman PhD, MRPharmS, an internationally recognized expert in the
science and business of adhesions, who has been making important product
and scientific contributions to the field since 1987, most recently
with Synechion, a consulting company he founded in 1996.
Contact:
Dr. David Wiseman, President
KevMed™, LLC
15305 Dallas Parkway, Suite 300, Addison, TX 75001
972 931 5596
david.wiseman@kevmed.com
Original press release here



