PainShield MD is an innovative treatment for pelvic and abdominal pain
including pain associated with the:
|  |
|
|
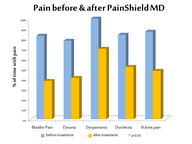
In a clinical study (see below) involving 19 patients with severe
chronic pelvic pain, interstitial cystitis or other conditions,
PainShield MD significantly (p<0.05) reduced:
| Reductions in the frequency of painful urinary episodes, sacro-iliac
joint pain, painful intercourse (dyspareunia), rectal pain, and pain
resulting from prolonged sitting were also noted. Some patients reported
a decrease in pain-associated sleep disturbance as well as reduced
expenditures for pain and related medications (e.g. opioids). Patients
rated their overall response as:
|
___________________________________
References
1. Ustinova E et al. Neurourol Urodyn. 2010; 29:77 - Cross-talk and sensitization of bladder afferent nerves.2. Rodriguez,et al., J Urol. 2009 182:2123 - Evidence for overlap between urological and nonurological unexplained clinical conditions.
3. FitzGerald, et al. J Urol. 2012: 187:2113 - Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness.
4. Montenegro et al., Int J Clin Pract. 2008 62:263 - Physical therapy in the management of women with chronic pelvic pain.
5. West et al. Gastroenterol Clin North Am. 1992; 21:793 - Diagnosis and management of irritable bowel syndrome, constipation, and diarrhea in pregnancy.
Full prescribing information can be found on our Ordering Page
___________________________________
Clinical Studies or Presentations with PainShield MD in Pelvic Pain
#1 - "Reduction of chronic abdominal and pelvic pain, urological and GI symptoms using a wearable device delivering low frequency ultrasound"
Summary: PainShield MD, a portable, wearable ultrasound device was found to reduce pelvic, urological pain and related symptoms in 19 patients presenting with long−standing and refractory symptoms. Click here for full abstract (presented at the International Pelvic Pain Society Meeting, Chicago, 2012)
 |  |
| Click here or on thumbnail for clinical data. | Click here for a video summary of the clinical data. |
#2 "Reduction of Chronic Pelvic, Urological and GI Pain Using Wearable Therapeutic Ultrasound in Women with Extended Follow-Up to 17 Months"
Summary: The reduction of pelvic, urological and GI pain by
PainShield MD, a wearable ultrasound device, persisted in this follow-up
to 541 (17 months) days in 16 women from study #1 with long-standing and refractory symptoms. Click here for the full abstract (presented
at the International Pelvic Pain Society, Orlando, 2013)


Click on one of the thumbnails above for clinical data.
This study was also presented at the annual meeting of AAGL, Washington DC, November 13 2013: click here to view.
_____________________________________
#3 "Strategies for Treating Chronic Pelvic, Urological and GI Pain Using Wearable Therapeutic Ultrasound"
Summary: PainShield® MD, a wearable
ultrasound device, has now been used for over four years to treat
chronic pelvic, urological, neurological and GI pain. Several treatment
strategies have emerged to optimize clinical outcome. Click here for full abstract.(presented at the International Pelvic Pain Society Meeting, Chicago, 2014)
 Click on the thumbnail to view the poster.
Click on the thumbnail to view the poster.
_____________________________________
#4 Reduction of Chronic Pelvic, Urological and GI Pain Using
Wearable Therapeutic Ultrasound in Men: Initial Experience in eight men
Summary: This is the first report of the use of this novel device
in men with pelvic and abdominal pain, and extends similar findings made in
women. PainShield MD, a wearable ultrasound device was
found to reduce pelvic, urological pain and related symptoms in 7/8 male patients
presenting with long−standing and refractory symptoms. Click here for the full abstract.
(presented at the Annual Meeting of the International Pelvic
Pain Society Meeting, San Diego, 2015)
 Click on the thumbnail to view the poster.
Click on the thumbnail to view the poster.
_____________________________________