#3 "Strategies for Treating Chronic Pelvic, Urological and GI Pain Using Wearable Therapeutic Ultrasound"
(presented at the Annual Meeting of the International Pelvic Pain Society Meeting, Chicago, Oct. 23-26, 2014, Poster 6)
David Wiseman, BSc, PhD, MRPharmS, KevMed LLC, Addison, TX

Click on the thumbnail above to view the poster with latest clinical data.
Summary
PainShield® MD, a wearable ultrasound device, has now been used for over four years to treat chronic pelvic, urological, neurological and GI pain. Several treatment strategies have emerged to optimize clinical outcome.
Objective
To describe strategies for treating chronic pelvic, urological, neurological and GI pain using wearable therapeutic ultrasound.
Methods
Patients: Patients undergoing treatment with PainShield MD for chronic pelvic, urological, neurological and GI pain.
Diagnoses: Adhesions, bowel obstruction, endometriosis, IBS, interstitial cystitis/ bladder pain, chronic pelvic pain, pudendal or other neuralgia, sacroiliac joint pain.
Treatment: Treatment consists of wearing a patch-mounted ultrasound transducer for 1-2 sessions/day. Each session consists of 13 alternating periods (30 minutes) of active and inactive ultrasound energy delivery.
Ultrasound: Frequency: Low frequency - 90kHz; Power: Low intensity - 0.4W (70mW/cm2) ;Wave type: Surface acoustic wave
Energy Transfer: < 4 cm below the surface with radius of ~ 10 cm
Strategy Capture: Interviews were conducted with patients using PainShield MD regarding their pattern of use of the device. General descriptive strategic outlines were drafted.
Patient Selection
History of chronic pelvic, urological, GI or related pain with:
- Suspected pelvic floor dysfunction and muscle spasm
- No or little response to other treatment
- Exclusion of other addressable causes from full-work up
- Side effects from opioid use
Consider especially candidates for:
- Neurostimulator implant
- Nerve block or ablation
- Opioid therapy
- Hysterectomy for pain
- Adhesiolysis only for pain
- Other invasive procedure for pain
Exclusions and Precautions
- Ultrasound is contraindicated in patients with InterStim® neurostimulators. Consult labelling for similar devices.
- Use with caution near meshes, IUDs and other implants
- Review other contraindications and precautions
Particularly good candidates may be those who:
- Respond to physical therapy but with relapse between visits
- Respond to non-wearable ultrasound
- Obtain some relief from heating packs or warm baths
- Use the device in the context of multidisciplinary treatment
- Have poor access to specialized physical therapy
Placement Strategies
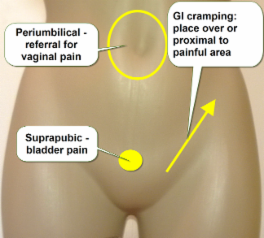 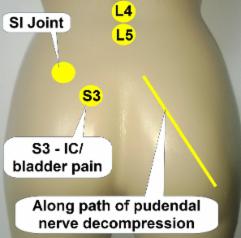 |
1. Place over or close to painful area
2. Place proximal along innervation path (S3, L4, L5, side or central)
3. Place on muscles in spasm (with advice of PT)
4. Place along referral pathway
5. In allodynia, mask adhesive & fix with self adherent bandage
Timing Strategies
1. Aim to keep muscles out of spasm: Start soon after successful PT
2. Begin treatment 1-2 hours before onset of pain if predictable, e.g.
- pain always/usually occurs around 10 pm
- pain always/usually occurs when eating
- pain always/usually occurs at time of regular bowel movement
- pain always/usually occurs during car journey
3. Apply after intercourse or when an “aura” signals a flare-up
Frequency of Use
1. Use for up to two sessions (6.5 hours each) per 24 hour period
2. Once effect occurs, taper frequency & treatment length appropriately
3. Relief usually extends beyond session time
Optimization
1. During optimization period, focus on one area at a time for 2-3 days.
2. Onset of relief: within hours to 14 days for ~90% of responders. Approximately 75-80% patients respond to treatment.
Patient Monitoring
1. Monitor patient progress, preferably using a pain diary – a free diary is available at: www.kevmed.com/PainDiary.html
2. For patients responding to treatment, taper down opioids and other medications under supervision
3. Counsel responsive patients to increase level of physical activity gradually and under medical / PT supervision
4.
Advise patients to report unusual symptoms such as swelling, or redness
that is more than transient, and to stop using device
5. A few
patients, especially those with a neuropathic component, report
“burning” or “painful” sensations on first use. If this occurs, reduce
the session length or distance the patch from the original site until
these sensations do not occur. Gradually increase session time, or patch
proximity to the original site.
Conclusion
Using the strategies described, PainShield MD wearable ultrasound can be integrated into almost any program to treat chronic pelvic and related pain.
Disclosure
KevMed, LLC. distributes PainShield MD Wearable Therapeutic Ultrasound.
Acknowledgement
We thank the many patients whose experiences have contributed to this work.
Copyright (C) 2015 KevMed, LLC.